Trends in benzene inverse sandwich complexes of the alkaline-earth metals Mg, Ca, Sr and Ba
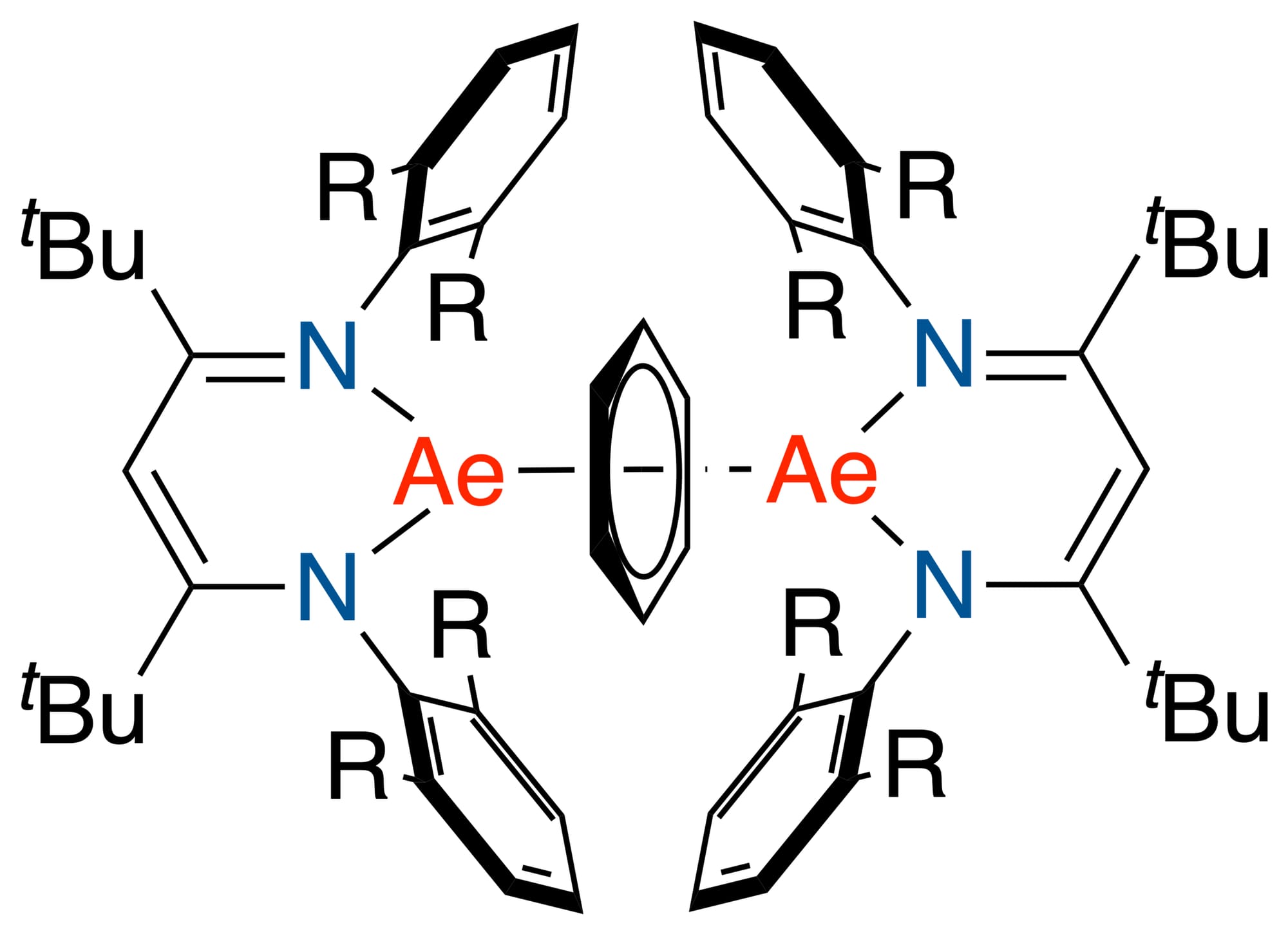
Mechanochemical reduction of β-diketiminate (BDI) barium iodide precursors with K/KI resulted in the first bariuminverse sandwich complexes containing the benzene dianion in yields of up to 54%. This most challenging isolation ofhighly reactive (BDI)Ba-(C6H6)-Ba(BDI) complexes, completes the family of heavier benzene inverse sandwichcomplexes and allows for a comparison of trends in the series from Mg, Ca, Sr to Ba. Syntheses, stabilities, structures,electronic states and reactivities of the full range are compared. Crucial for isolation of the Ba inverse sandwichcomplexes are the tBu-substituents in the ligand backbone which push the bulky aryl rings towards the large Ba metalcations. These secondary Ba···(-Ar) interactions result in an unexpected high stability. Another trend is found for thering puckering in the bridging benzene2- dianion which steadily increases from Ba to Mg. DFT calculations show thegeneral ionic character of (BDI)Ae-(C6H6)-Ae(BDI) complexes (Ae = Mg, Ca, Sr, Ba) and reveal only small energydifferences between closed-shell singlet or open-shell triplet states. The most reactive (BDI)Ba-(C6H6)-Ba(BDI)complexes could be considered the first BaI synthons. They reduce a range of polyaromatic hydrocarbons, H2 or evenconvert (BDI)MgI precursors into well-known (BDI)Mg-Mg(BDI) complexes. Reactions with heavier (BDI)AeI (Ae = Ca,Sr) gave (BDI)Ae-(C6H6)-Ae(BDI) and (BDI)BaI.